Application of Biosecurity Strategies for the Control of MAS (Motile Aeromonas Septicemia) in Tubifex Worms (Tubifex sp.) for Larval Catfish (Pangasius sp.) Culture
Main Article Content
Amalia Putri Firdausi
Cecilia Eny Indriastuti
Nur Prasetyo Ari Surahman
Sheny Permatasari
Background: Catfish (Pangasius sp.) is one of the high-value freshwater aquaculture commodities in Indonesia. To ensure sustainable production, hatchery management plays a crucial role, as the larval and juvenile stages are the most vulnerable to environmental stress and disease outbreaks. During the larval phase (7–14 days post-hatch), Tubifex worms (Tubifex sp.) are commonly used as a natural feed due to their high nutritional content and digestibility.
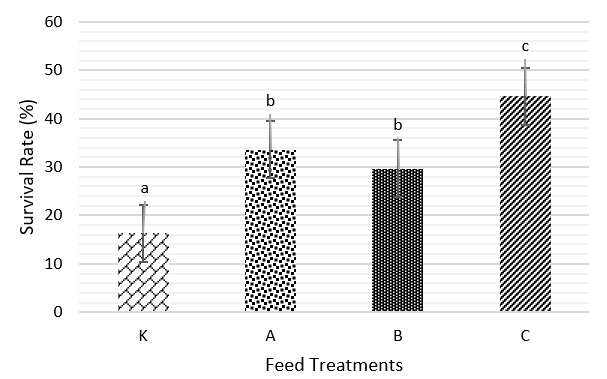
Aims & Methods: This study aimed to evaluate the effectiveness of formalin in suppressing Aeromonas hydrophila in Tubifex worms (Tubifex sp.), which are used as natural feed for larval catfish (Pangasius sp.). The research was conducted in two phases: in vitro and in vivo assays. The in vitro assay was carried out to determine the minimum inhibitory concentration (MIC) and inhibition zone of formalin against A. hydrophila. The in vivo assay consisted of two parts: a toxicity test of formalin on Tubifex worms and an evaluation of the effect of treated worms as feed on the survival of catfish larvae. A completely randomized design (RAL) was employed, consisting of four treatments with three replicates each: K (control—untreated Tubifex), A (400 ppm formalin immersion without rinsing), B (400 ppm formalin immersion with one rinse), and C (400 ppm formalin immersion with two rinses).
Result: The results demonstrated that a 400 ppm formalin concentration effectively inhibited the growth of A. hydrophila. Treatment C (two rinses following immersion in 400 ppm formalin) significantly reduced the toxic effects of formalin on the Tubifex worms used as natural feed. Consequently, this treatment led to an improvement in the survival rate of catfish larvae, reaching 44.6 ± 11.5% over a 14-day rearing period.
Abdi, R., Setyowati, D. N., & Mukhlis, A. (2022). Pengaruh penambahan ekstrak daun jeruju (Acanthus ilicifolius) dengan dosis berbeda pada pakan terhadap system imun udang vaname (Litopenaeus vannamei) yang diinfeksi Vibrio parahaemolyticus. Jurnal Perikanan, 2(1), 33-44. https://doi.org/10.29303/jp.v12i1.271
Austin, B; Austin, D. A. (2016). Bacterial Fish Pathogens: Disease of Farmed and Wild Fish (6th ed.). Springer
Badan Standarisasi Nasional. (2000). SNI 01648. 1-2000 Induk ikan patin siam (Pangasius hypophthalmus) kelas induk pokok (Parent Stock). hlmn 9.
Cabello, F. C. (2006). Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environmental Microbiology, 8(7), 1137-1144. https://doi.org/10.1111/j.1462-2920.2006.01054.x
Chinabut, S., Limsuwan, C., & Sangjan, M. (1988). Formalin: its toxicity to Aeromonas hydrophila, planktons and degradation. Network of Aquaculture Centres in Asia. https://openknowledge.fao.org/items/e5ed90b6-bee3-4f4f-9c20-69c8ec5ce9da
Fitriadi, B. R., & Putri A. C. (2016). Metode-Metode Pengurangan Residu Pestisida pada Hasil Pertanian. Jurnal Rekayasa Kimia dan Lingkungan, 11(2), 61-71. https://doi.org/10.23955/rkl.v11i2.4950
Floyd, R. R., & Pouder, D. B. (2023). Use of Formalin to Control Fish Parasites, VM 77. IFAS Extension. University of Florida
Harikrishnan, R., & Balasundaram, C. (2005). Modern trends in Aeromonas hydrophila disease. Reviews in Fisheries Science, 13(4), 281-320.
Hatmanti, A., Nuchsin, R., & Dewi, J. (2009). Screening bakteri penghambat untuk bakteri penyebab penyakit pada budidaya ikan kerapu dari perairan Banten dan Lampung. Jurnal Makara Sains, 13(1), 81-86.
Jaya, R. (2011). Hubungan parameter kualitas air dalam budidaya ikan nila [skripsi]. Universitas Negeri Musamus. Merauke
Kementerian Kelautan dan Perikanan. (2020). Produksi perikanan [internet]. [diakses 2024 Januari 14]. https://statistik.kkp.go.id.management.
Khairuman, K; Amri, K. (2002). Membuat Pakan Ikan Konsumsi. PT. Agro Media Pustaka. Depok.
Leal, J., Neves, M. G. P. M. S., Santos, E. B. H., & Esteves, V. I. (2018). Use of formalin in intensive aquaculture: Properties, application and effects on fish and water quality. Reviews in Aquaculture, 10(2), 281-295. https://doi.org/10.1111/raq.12160
Lestari, N. W., Budiharjo, A., & Pangastuti, A. (2016). Bakteri heterotrof aerobic asal saluran pencernaan ikan sidat (Anguilla bicolor bicolor) dan potensinya sebagai probiotik. Jurnal Bioteknologi, 13(1), 9-17.
Nikoskelainen, S., Ouwehand, A. C., Bylund, G., Salminen, S., & Lilius, E. M. (2001). Protection of rainbow trout (Oncorhynchus mykiss) against furunculosis by Lactobacillus rhamnosus. Aquaculture, 198(3), 229-236. https://doi.org/10.1016/S0044-8486(01)00593-2
Sacher, R. A; McPherson, R. A; Campos, J. M; Widman, F. K. (2000). Widmann's clinical interpretation of laboratory tests. FA Davis.
Sanoesi, E. (2008). Penggunaan ekstrak daun pepaya Carica papaya Linn terhadap jumlah sel makrofag pada ikan mas Cyprinus carpio yang terinfeksi bakteri Aeromonas hydrophila. Jurnal Penelitian Perikanan, 11(2).
Santi, A. U. P. (2017). Analisis kandungan zat pengawet boraks pada jajanan sekolah di SDN Serua Indah 1 Kota Ciputat. Holistika Jurnal Ilmiah PGSD, 1(1), 57-62.
Saptarini, N., Wardati, Y., & Supriatna, U. (2011). Deteksi formalin dalam tahu di pasar tradisional purwakarta. Sains dan Teknologi, 12(1), 37-44.
Setiadi, Wadjdy, E. F. (2021). Karakterisasi dan identifikasi molekuler Aeromonas hydrophila hasil postulat koch pada ikan nila Oreochromis niloticus. Buletin Teknik Litkayasa Akuakultur, 19(1), 53-59.
Sherif, A. H., Farag, E. A. H., & Mahmoud, A. E. (2024). Temperature fluctuation alters immuno-antioxidant response and enhances the susceptibility of Oreochromis niloticus to Aeromonas hydrophila challenge. Aquaculture International, 32, 2171–2184. https://doi.org/10.1007/s10499-023-01263-9
Surjowardojo, Susilorini, T. E., & Sirait, G. R. (2015). Daya hambat dekok kulit apel Manalagi Malus sylvestris Mill Terhadap Pertumbuhan Staphylococcus aureus dan Pseudomonas sp. penyebab mastitis pada sapi perah. Jurnal Ternak Tropika, 16(2), 40–48.
Toy, T. S. S., Lampus, B. S., & Hutagalung, S. P. (2015). Uji daya hambat ekstrak rumput laut Gracilaria sp. terhadap pertumbuhan Bakteri Staphylococcus aureus. Jurnal e-GiGi, 3(1), 153–159.
Widanarni, W., Lidaeni, M. A, & Wahjuningrum, D. (2010). Pengaruh pemberian bakteri probiotik Vibrio SKT-b dengan dosis yang berbeda terhadap kelangsungan hidup dan pertumbuhan larva udang windu (Penaeus monodon) Fab. Jurnal Akuakultur Indonesia, 9(1), 21–29.
Amalia Putri Firdausi , School of Vocational Studies, IPB University, Indonesia
Department of Technology and Management of Applied Aquaculture, School of Vocational Studies, IPB University, Indonesia
Cecilia Eny Indriastuti , School of Vocational Studies, IPB University, Indonesia
Department of Technology and Management of Applied Aquaculture, School of Vocational Studies, IPB University, Indonesia
Nur Prasetyo Ari Surahman , School of Vocational Studies, IPB University, Indonesia
Department of Technology and Management of Applied Aquaculture, School of Vocational Studies, IPB University, Indonesia
Sheny Permatasari , School of Vocational Studies, IPB University, Indonesia
Department of Technology and Management of Applied Aquaculture, School of Vocational Studies, IPB University, Indonesia